Conventionally, there are two methods for creating flakes. One of them is crushing it after forming metal foil as a precursor to get flakes. The other one is making a sphere-formed particle as a precursor by atomization method, changing it to flakes by rolling it when it is crushed. Both methods are complicated because we need to melt them to produce precursor and crush them. Another problem is that it is not possible to make flakes from hard metal elements.
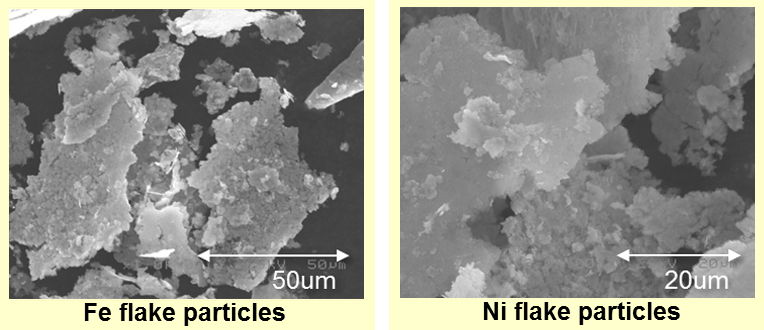
By the composition method we developed newly, we can produce flakes of metallic elements, such as Fe, Ni, and Cu. Small production is possible. The shape control is possible to a certain degree. Theoretically, making flakes of almost all kinds of elements and alloy composition will be possible. It is similar as the size control.

The representative application example of needle-shaped nanoparticles is magnetic particles of magnetic recording medium. In this case, we produce needle-shaped precursor of iron hydroxide, such as goethite and lepidocrocite. Moreover, we produce metal particles by hydrogen reduction of them. By adding other elements except Fe, when we produce precursor, control of the coercive force, aspect ratio and control of the thickness of oxidation are possible by changing them to metal alloy particles after reduction. In this method, shape control of the metal powder is limited depending on the range of the crystal shape variety of the hydroxide. The composition of needle-shaped nanoparticles of Cu was found difficult.
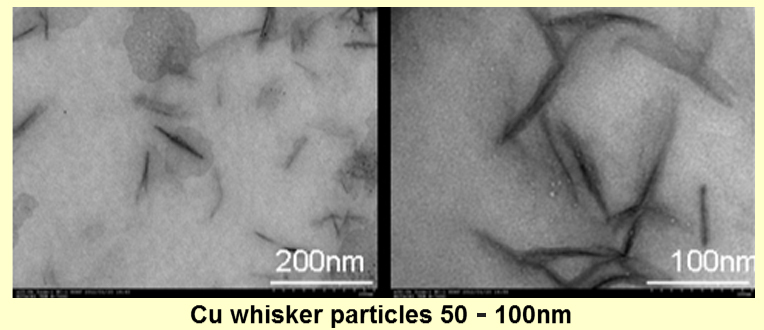
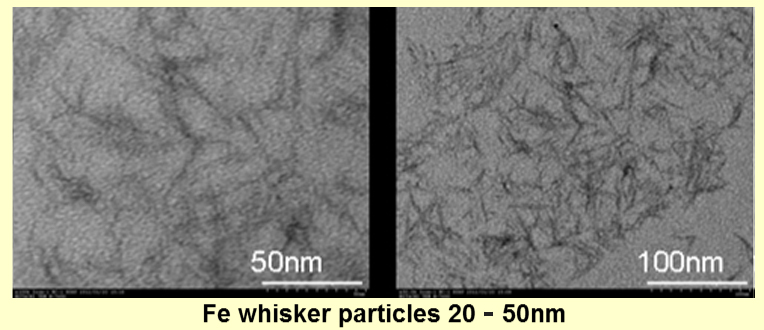
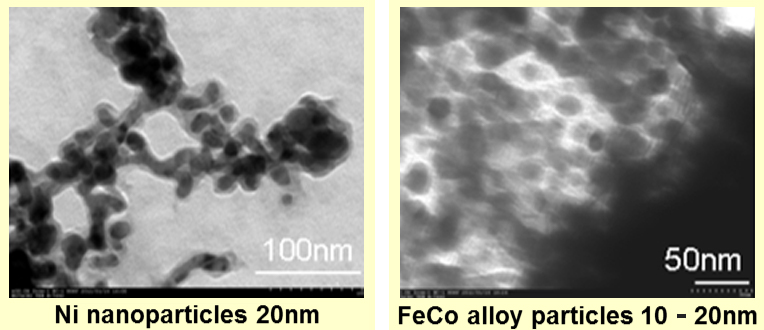
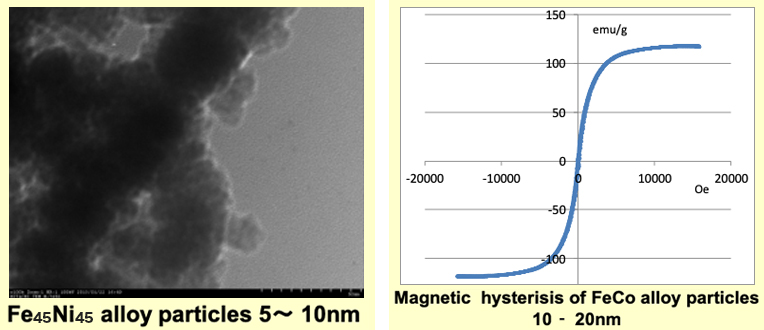
We also can compose needle-shaped or spherical magnetic particles by our new composition method, which does not need to be made from the precursor at room temperature. Observed by TEM, we could compose metal magnetic particles of 5-50nm, although it depends on the kind of metal. Theoretically, the composition of the multi-element-based magnetic particles is also possible by this method. We can offer them cheaply in the mass production using this method.

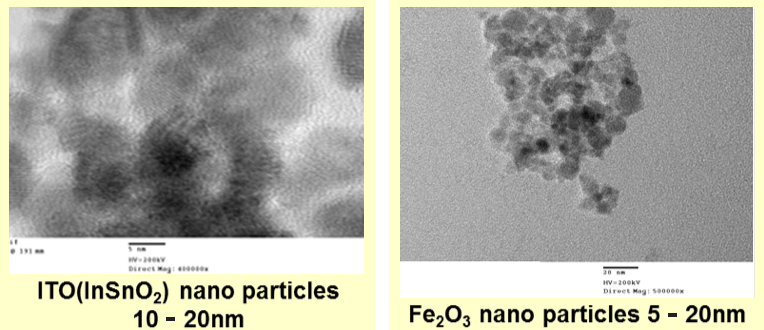
We showed below the example of nanoparticles of the metal oxide produced by our new method. The size of the ITO particles is around 10-20nm, which is extremely small. In addition, the size of SnO powder is micron as the secondary particle, the primary particle of this is produced by agglutinating SnO nanoparticles to 100nm size, and the powder showed below is more aggregated one.
The size of iron oxide is around 10-20nm. In this way, our method of producing metal oxide nanoparticles is not limited by the elemental kind. Although the kind of metal is not limited, it is necessary to choose the best method depending on the elements.

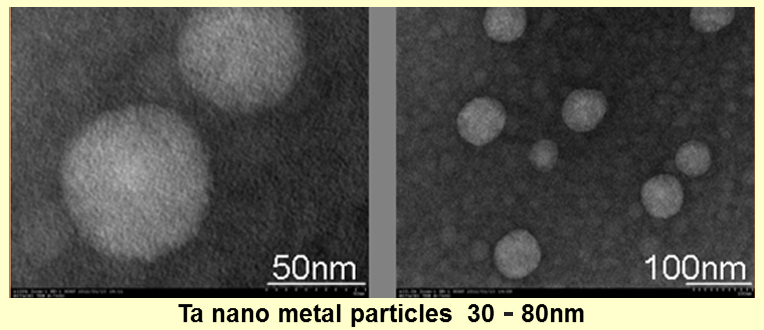
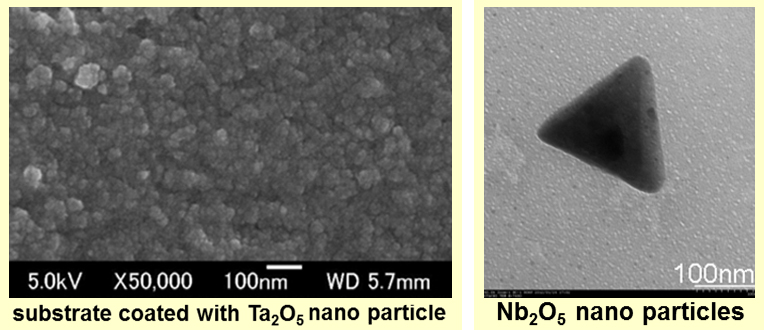
We are thinking one new method, which can mass produce nanoparticles at low cost, with which we can produce metal nanoparticles of 30-50nm of Ta, Nb, and Mo. Similarly, in the case of Ta, Mo, we can get very dense films as the following SEM photographs when metal oxide nanoparticles of Ta, Mo of dozens of nm produced by our new method is used.
We will examine the limit of the characteristic control of the particle composition of this method when the elements are changed by using various elements and various alloy composition.With this method, we can offer them very cheaply and easily in the stage of mass production. Observed in TEM, Nb205 made by our new method formed the crystal particles of equilateral triangle like the following picture.

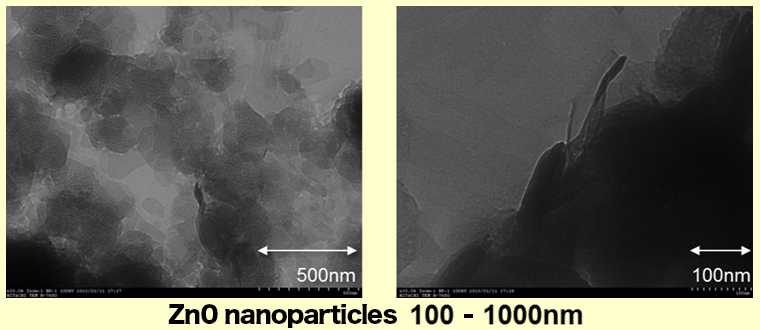
In our new composition method, we can manufacture thin ZnO particles of around 5-10nm as follows. Those size of ZnO is around a couple of 100nm.
Theoretically producing compound oxide with other elements is easy because it is not the coprecipitation method. This is the method that we can offer it very cheaply not only in the trial manufacture, but also in the mass production.
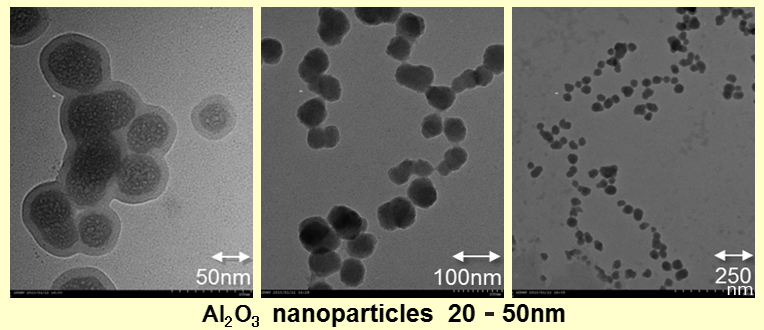
In our new composition method, we can manufacture around 50nm Al2O3 particle as follows. Theoretically, producing compound oxides with other elements is easy. This is the method with which we can produce nanoparticles stably not only in the trial manufacture, but also in the mass production, and can offer cheaply. (The following picture is transparent because it’s dispersed in water.)
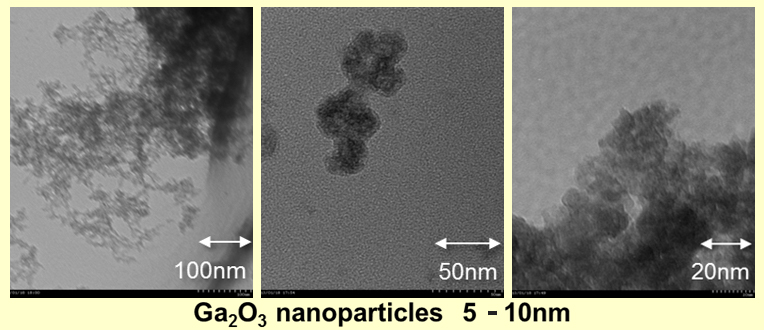
Generally, the Ga2O3 particles are produced by forming hydroxide of Ga metal salts, such as l sulfate, with alkali and heating it. It is difficult to make very thin powder by such a method. Not only that, the composition is limited to the range in which hydroxide can be coprecipitated.
The size of Ga2O3 nanoparticles made by our new composition method is around 5-10nm, very small as follows, and transparent when it is dispersed in water. This is the method by which we can offer it very cheaply not only in the trial manufacture, but also in the mass production.
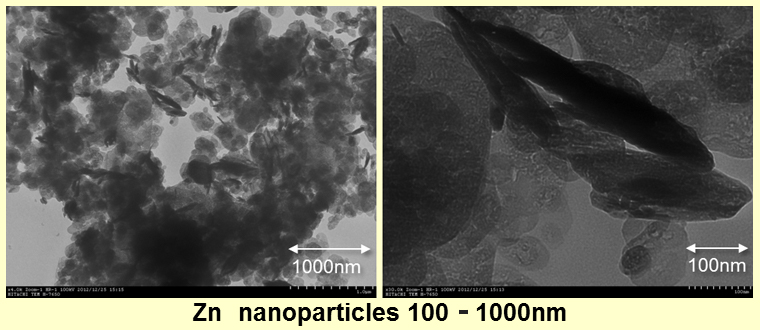
Generally, the Zn particles are produced by gas atomization method and disk atomization method. Therefore, the limit of the particle size was at smallest several microns. A particle grows up because it sinters during floating under vacuum by the evaporation method, so it was difficult to control size and particle size distribution.
In the new composition method that we made lately, the following size of Zn nanoparticles of 100-300nm was confirmed. Theoretically, alloying other elements is also easy if we use this method. We are going to check the limit of the characteristic control with various elements. This is the method with which we can offer them very cheaply not only in the trial manufacture, but also in the mass production.
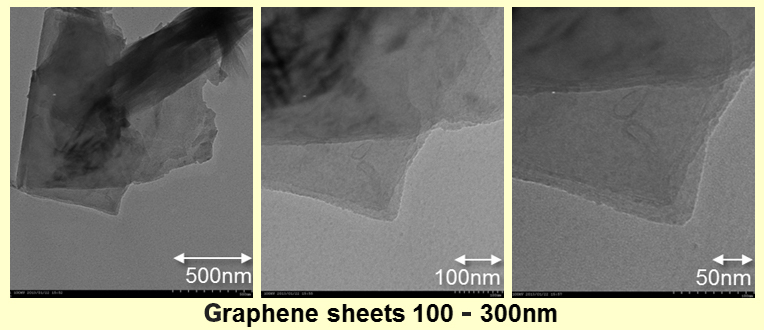
The Graphene is produced experimentally by various methods, but still now, it is difficult to mass-produce at low cost.
There is a method using plasma and CNT as a raw material. However, either of them cannot enable us to mass-produce it at low cost.
As the following images, we crushed natural black lead simply, and found Graphene distributed by one of our new methods. You can see the yellow colored dispersion liquid. When the density is raised, it becomes aggregate condition microscopically. So, we are now examining the crush and the dispersion.
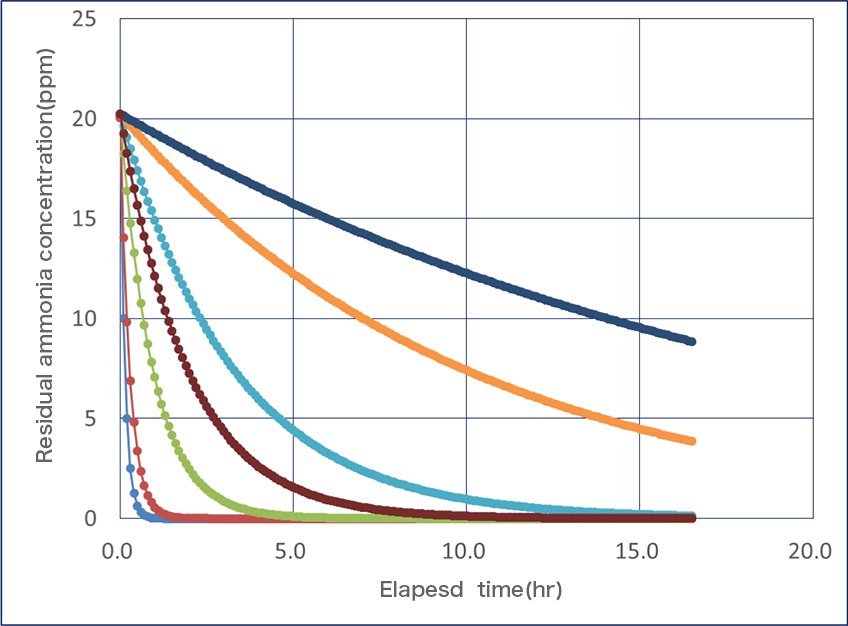
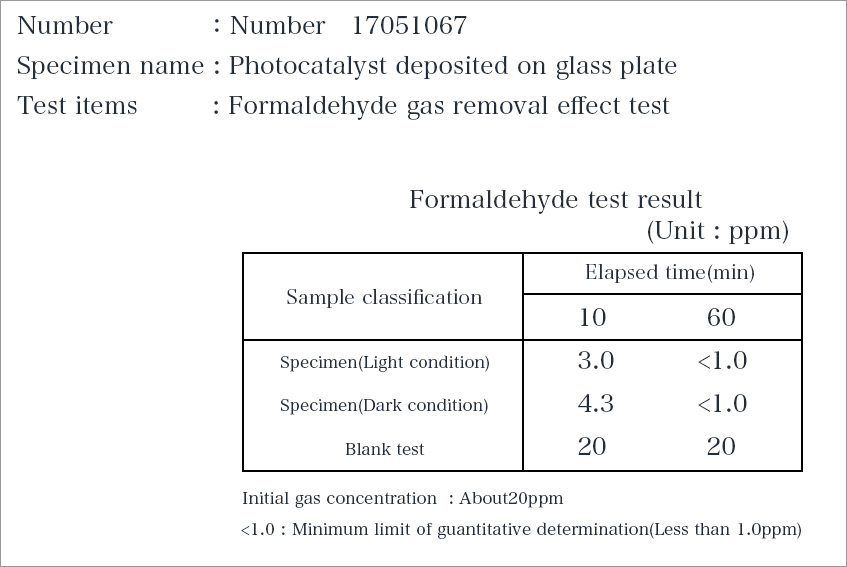
Result of a measurement of Japan Food Research Laboratories (JFRL)
The following graphs compare the ammonia decomposition speed when the quantity of the supported catalyst is decreased. It turned out that the speed greatly changes when the supported catalyst is reduced. Even a smelly person does not have more ammonia levels than 5ppm, which means deodorization of sportswear, bed clothing and the restroom can be possible by using our catalyst.